Patient safety in medical research has become a pivotal concern in the evolving landscape of biomedical studies. As research funding cuts threaten the continuity and integrity of clinical trials, maintaining robust oversight is more critical than ever. The role of institutional review boards (IRBs) is essential in ensuring that ethical standards are upheld while protecting the rights of participants involved in medical trials. These boards evaluate not only the feasibility of research proposals but actively monitor adherence to ethical practices, which enhances the overall trustworthiness of scientific findings. With the looming impact of NIH funding cuts and regulatory disruptions, the future of patient safety in medical research hangs in the balance, necessitating urgent attention and advocacy for maintaining ethical oversight.
The concept of safeguarding patient welfare during scientific inquiries, often referred to as participant protection in clinical studies, is increasingly recognized as a cornerstone of ethical research practices. Amidst discussions surrounding budget reductions in medical research funding, the spotlight is cast on the critical functions played by regulatory bodies, such as institutional review boards (IRBs), in upholding the safety of volunteers in these trials. With growing concerns over the ethical implications and potential hazards of experimental studies, ensuring rigorous clinical research oversight is imperative. The future trajectory of health research depends on robust federal support and adherence to established ethical standards, all pivotal to fostering public confidence in new medical solutions. In this shifting environment, the dialogue surrounding the significance of ethical practices and governance in medical studies remains essential.
The Impact of Research Funding Cuts on Patient Safety
The cuts to research funding, particularly in federal grants, have a direct impact on the capacity to ensure patient safety in medical research. With significant funding from the NIH, institutions like Harvard rely on these resources to support various safety protocols outlined by IRBs. These boards are critical in reviewing and approving research studies, ensuring that the safety and welfare of all participants are prioritized. When funding is reduced, institutions struggle to maintain the necessary oversight and resources required to conduct ethical medical research, thereby increasing risks for clinical trial participants.
Moreover, funding cuts lead to a ripple effect that hampers the overall infrastructure of clinical research. As resources dwindle, institutions may face difficulties in adhering to comprehensive safety evaluations, which can lead to gaps in patient protections. The inability to maintain thorough reviews underlines the crucial role that consistent funding plays in fostering a safe research environment. Institutions may find themselves overstretched, unable to fulfill their IRB obligations, thereby placing an undue burden not only on patients involved in existing studies but also on the integrity of future medical research.
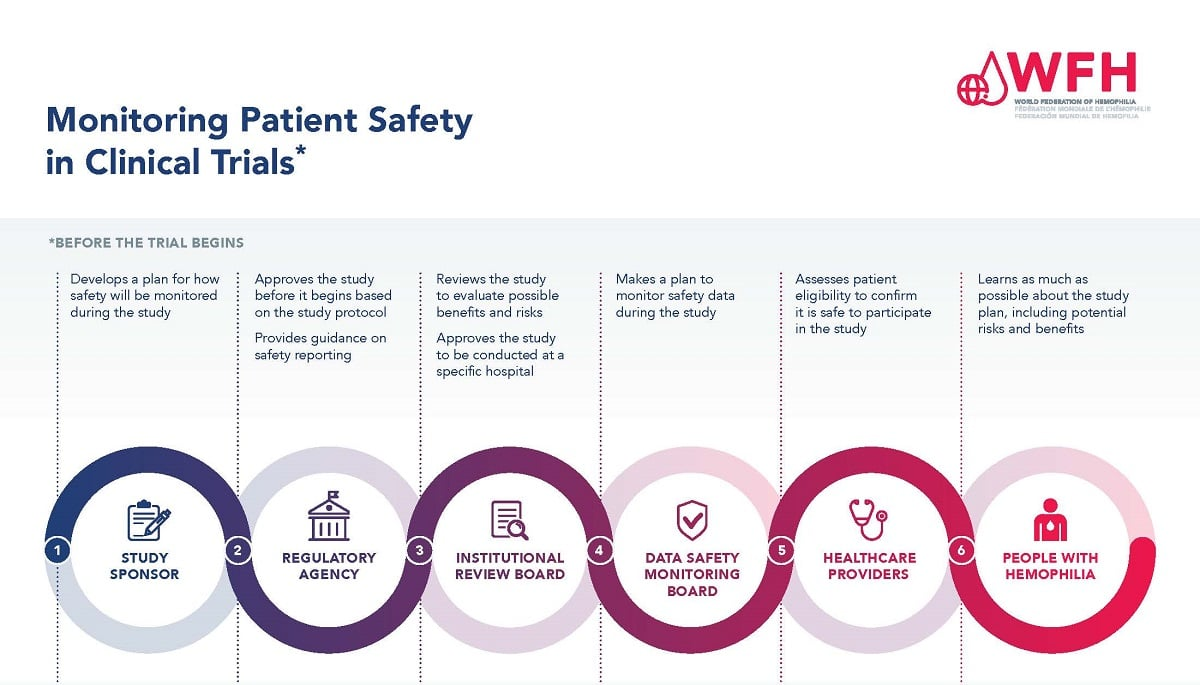
The Essential Role of Institutional Review Boards (IRBs) in Medical Ethics
Institutional Review Boards, or IRBs, are pivotal in the landscape of clinical research, serving as gatekeepers dedicated to safeguarding participant rights and ethics throughout the research process. Their role involves rigorous examination of research proposals, ensuring comprehensive plans for informed consent, risk assessment, and participant safety are thoroughly evaluated. This oversight is not just a bureaucratic step; it’s an ethical obligation that has emerged from historical lessons about the importance of human rights in medical research. Without IRBs, there would be no structured mechanism to protect participants from potential dangers associated with clinical trials.
The ethical landscape of medical research has evolved significantly, thanks to the critical work of IRBs. Given their role in addressing ethical issues, IRBs can assess the implications of research on participants, which includes evaluating potential adverse effects related to studies. The need for ethical vigilance is particularly stressed in light of past atrocities, making IRBs not only essential for compliance but also for restoring public trust in medical research. By actively engaging with state and federal regulations, IRBs ensure that clinical research is conducted with the highest ethical standards, fostering a climate where patient safety is paramount.
Research funded by NIH and similar organizations depends heavily on the oversight provided by these boards. As institutions face funding cuts, the burden falls on IRBs to adapt quickly, often leading to a compromise in the thoroughness of their review processes. This creates a concerning gap in ethical oversight, as IRB members might be overworked and under-resourced, which can lead to rushed approvals. The essential role of IRBs, particularly in the wake of funding challenges, underscores the critical importance of sustained support for these essential watchdog bodies to ensure the continued effectiveness of clinical research oversight.
The Consequences of Inadequate Support for Clinical Trial Oversight
In light of recent federal funding cuts, there are dire consequences for clinical trial oversight and ultimately for patient safety in medical research. With the halt of NIH funding and the suspension of various grants, many IRBs find themselves facing resource strain. This situation threatens the integrity of research oversight as IRBs may lack the personnel needed to evaluate studies adequately. An underfunded oversight system can increase the likelihood of ethical violations, thus jeopardizing participant safety and eroding public trust in the research enterprise.
As patient safety becomes compromised due to insufficient oversight, the overall advancement of medical knowledge is also put at risk. The cancelled or delayed studies stemming from these funding cuts mean that potential breakthroughs in treatment and understanding diseases are slowed, which affects not only the immediate participants but also the broader patient population reliant on medical advancements. With each compromised study, the trust gap between the public and medical researchers widens, potentially deterring future participation in clinical trials—a situation that can have long-lasting implications for medical research.
Understanding the Ethics of Clinical Research
Ethics play a fundamental role in all phases of clinical research, dictating how participant safety is prioritized and how risks are presented to those involved. Since their inception, ethical guidelines in research have been crucial in shaping practices that respect individual rights. The actions taken in past unethical studies have led to strict regulations that guide current research protocols, emphasizing the importance of informed consent. These ethical frameworks are responsible for ensuring that patients understand the implications of their involvement through clear explanations of the research process and potential risks.
The ethical obligations in clinical research also extend to creating a supportive framework where participants feel safe to voice their concerns. When institutions face constraints due to funding cuts, the ethical dimensions of informed consent and participant safety become even more critical. Researchers are ethically bound to uphold respect for their participant’s rights and ensure transparent communication regarding study-related risks and benefits. Thus, maintaining a robust ethical oversight system—backed by adequate funding—is essential not just for compliance but for the moral duty owed to those who volunteer their time and health for the advancement of medical science.
The NIH Funding Impact on Research Ethics and Oversight
NIH funding has traditionally been a cornerstone for supporting research initiatives aimed at enhancing patient safety and ethical conduct in medical studies. The provisions established through these funds enable institutions to implement necessary safeguards, such as rigorous IRB reviews, extensive training for key personnel, and comprehensive data monitoring strategies. Without this financial support, institutions may struggle to maintain the critical infrastructure necessary for ethical oversight, leading to potential compromises in patient safety.
Additionally, the presence of NIH funding brings with it a set of stringent expectations for compliance with ethical standards. These expectations serve as a powerful motivator for institutions to adhere to the highest ethical conduct in research. The absence of such funding not only jeopardizes patient safety but also creates an environment where it may be easier to overlook ethical considerations. Hence, maintaining robust NIH funding is crucial for fostering both innovation and ethical medical research practices.
Navigating the Landscape of Medical Trials and Ethics
Medical trials are characterized by their complexity and the inherent need for strict ethical standards to protect participants. As various stakeholders engage in clinical research, ethics becomes a guiding principle that framers of these trials must diligently uphold. This necessity is underscored by historical events demonstrating the drastic consequences of ethical oversights. As medical research evolves, so too must the understanding of ethical implications that come with innovative treatments and theories, ensuring that participants can rely on frameworks that prioritize their well-being.
In navigating this landscape, collaboration between IRBs, researchers, and regulatory bodies is essential to ensure that all ethical concerns are effectively addressed. By establishing robust communication channels, stakeholders can work together to anticipate and resolve potential ethical dilemmas. This collective effort not only strengthens patient protection but also enhances the credibility of medical trials, reassuring participants that their rights and safety remain at the forefront of research initiatives.
The Importance of Transparent Communication in Research
Transparency is critical in clinical research, impacting everything from participant recruitment to informed consent processes. Clear communication fosters trust between researchers and participants, essential for encouraging individuals to take part in medical trials. Participants must understand the purpose of a study, what their involvement entails, potential risks, and any benefits they might hope to achieve. When funding cuts limit resources for educational outreach, this transparency can suffer, limiting the depth of understanding among potential study participants.
Moreover, transparent communication doesn’t solely benefit participants; it also plays a vital role in ensuring accountability among researchers. Institutions must convey the measures they are taking to safeguard the rights and welfare of study participants, especially when resources are stretched thin due to funding constraints. By prioritizing transparency, researchers can maintain ethical standards, reassure participants, and avoid the pitfalls of mistrust that can arise from ambiguity or lack of information.
Fostering Public Trust in Medical Research After Scandals
Public trust is an essential pillar for the successful continuation of medical research. Trust allows for participation in clinical trials, which in turn fuels medical advancement. However, past incidents of unethical human experimentation have led to widespread skepticism, making transparent ethical practices even more crucial in today’s landscape. To regain this trust, institutions must actively engage with the communities they serve, demonstrating a commitment to ethical oversight through robust IRB systems and transparent communication.
Additionally, addressing past breaches of trust, such as those stemming from unethical experiments, can create pathways for improving public perception. Institutions must be open about steps taken to ensure ethical standards are met and the safety of participants is prioritized. Rebuilding trust requires a continuous effort to involve community voices in discussions about research and its ethics, ensuring that future studies uphold the highest ethical standards and genuinely respect participant autonomy.
The Role of Community Engagement in Clinical Research
Community engagement is increasingly recognized as a cornerstone of ethical clinical research, ensuring that research practices are aligned with the values and needs of those involved. By actively involving community members in the research process, institutions can better understand the concerns and needs of potential participants. This engagement helps to demystify the research process, clarifying intentions and methodologies, thereby enhancing participant recruitment and retention while fostering a sense of shared responsibility in the research endeavor.
Furthermore, community engagement serves as a feedback mechanism that can guide researchers in designing studies that are more relevant and considerate of participant welfare. Through open dialogue, community members can voice their thoughts on ethical concerns or suggestions regarding studies, which translates to more ethically sound practices. This collaborative approach not only enhances the ethical landscape concerning patient safety in medical research but also works to build trust in the broader research community.
Frequently Asked Questions
How do funding cuts impact patient safety in medical research?
Funding cuts, especially to organizations like the NIH, can severely disrupt systems that ensure patient safety in medical research. When funding is reduced, essential oversight functions by institutional review boards (IRBs) are hindered, delaying studies and risking the safety of participants. With fewer resources, the capability to monitor and support ethical research practices diminishes, leading to potential harm and loss of public trust in clinical trials.
What role do IRBs play in ensuring patient safety in medical research?
Institutional Review Boards (IRBs) are vital for protecting patient safety in medical research by reviewing study protocols to ensure compliance with ethical standards. They assess risks, obtain informed consent, and monitor adverse events, thus acting as a check on research practices. Their oversight helps mitigate risks to participants, ensuring that their rights and welfare are safeguarded throughout the research process.
How does NIH funding impact safety measures in medical research?
NIH funding is crucial in sustaining safety measures for participants in medical research. It supports key processes like the IRB review, which ensures that studies are designed ethically and that patient safety is prioritized. Funding also facilitates training for researchers in ethics and oversight, reinforcing the infrastructure needed to keep research participants safe from harm.
What is the significance of ethics in medical trials related to patient safety?
Ethics in medical trials is essential for maintaining patient safety, as it establishes guidelines that govern research conduct. Ethical frameworks promote transparency and informed consent, ensuring participants are fully aware of risks and benefits. These guidelines are enshrined in laws and regulations shaped by historical abuses in research, highlighting their importance in protecting participants’ rights and well-being.
What challenges do funding cuts create for oversight systems in patient safety?
Funding cuts create significant challenges for oversight systems essential for patient safety in medical research. They can lead to reduced IRB functions, insufficient resources for monitoring studies, and delays in the approval of new clinical sites. These setbacks can compromise patient safety efforts, increase risks for participants, and ultimately hinder the advancement of necessary medical therapies.
How does collaborative research contribute to patient safety in medical research?
Collaborative research enhances patient safety in medical research by pooling resources, expertise, and oversight capabilities across institutions. By working together, IRBs and research teams can share best practices, streamline review processes, and more effectively manage the ethical aspects of trials. This collaboration ensures that patient safety remains a top priority, even amidst challenges like funding cuts.
Why are public perceptions important for patient safety in medical research?
Public perceptions are critical for patient safety in medical research because trust in the research process encourages participation. Historically, breaches of ethics have led to skepticism; thus, maintaining transparency and showing commitment to participant safety are vital. When trust is bolstered through ethical practices and active community engagement, it not only protects participants but also ensures the continued success of medical research initiatives.
| Key Point | Details |
|---|---|
| Funding Cuts Impact | Funding cuts, specifically a $2 billion freeze by the Trump administration, disrupt patient safety and research oversight. |
| SMART IRB Role | SMART IRB facilitates oversight of multisite medical research, crucial for ensuring participant safety. |
| Importance of IRBs | Institutional Review Boards (IRBs) are vital for reviewing research proposals and safeguarding patient rights. |
| Ethical Oversight | IRBs act as a checks and balances system for ethical medical research, preventing potential harm to participants. |
| Public Trust | Historical abuses highlight the necessity of IRBs to protect public trust and ensure informed consent in research. |
| Consequences of Research Disruption | Halting studies can lead to serious risks for participants, increased public skepticism, and a negative impact on scientific research. |
Summary
Patient safety in medical research is critically at risk due to funding cuts, particularly the significant freeze on federal research grants that has disrupted oversight systems essential for safeguarding participants. Ensuring robust patient safety protocols through organizations like SMART IRB is vital for maintaining ethical standards and conducting safe research. When funding is slashed, it not only endangers the welfare of individuals involved in studies but also threatens the integrity of the entire research process by amplifying public mistrust. Therefore, protecting patient safety in medical research must remain a top priority.